Use of Lithium and its isotopes as tracers for groundwater salinization
Li-Lithium; H-Hydrogen; O-Oxygen
𝛿7Li & 𝛿18O helps in identifying the source of salinization and whether Li fraction is occurring in groundwater or not.
𝛿7Li & 𝛿18O helps in identifying the source of salinization and whether Li fraction is occurring in groundwater or not.
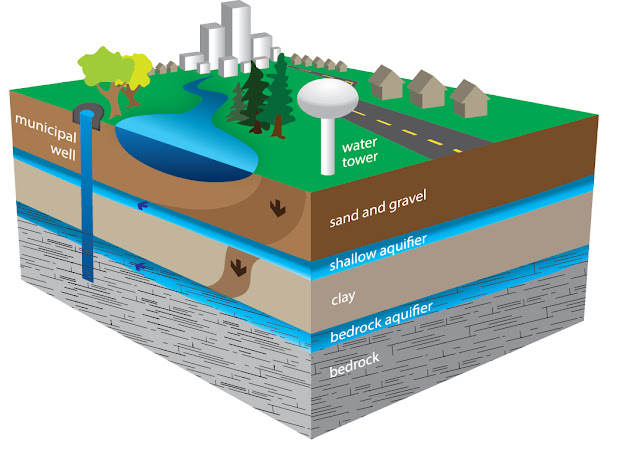
Due
to extensive exploitation of groundwater in the shallow aquifer process of
saltwater intrusion has occurred, as a result of which extraction from deep
groundwater started. Li, H, and O are used to make a check on this and know
about the hydrogeological conditions.
The
source of salinization was found to be brine water which formed as a result of
evaporation from seawater.
As
a result of the increase in demand for water, the depth of water extraction has
increased which gave rise to deterioration of groundwater quality mainly
because of salinization which is a common phenomenon in the coastal areas.
Moreover, climate change has a direct effect on groundwater quality as when the
ice in sea melts due to global warming, there is an increase in seawater level
which increases the pressure due to elevation and as a result water from the sea
shifts towards lower elevated groundwater making it saline.
Groundwater
samples can be divided into 4 types:
·
Fresh
G.W (TDS<1g/L) (TDS-Total Dissolves Solids)
·
Brackish
G.W (1<TDS<10g/L)
·
Saline
G.W (10<TDS<100g/L)
·
Brine
(TDS>100g/L)
Evaporation
is an important factor affecting the groundwater.
Shallow G.W samples with TDS>50 g/L and Cl concentrations more than seawater should be mixture of seawater and brine.
Shallow G.W samples with TDS>50 g/L and Cl concentrations more than seawater should be mixture of seawater and brine.
Deep
G.W samples with TDS<50g/L and Cl concentrations lesser than seawater should
be a mixture of deep fresh G.W with seawater or brine.
Increase
in Li isotope concentration:
1.
Brine
mixing with seawater
2.
Fractionation
from ion exchange in clay minerals while mixing of brine and seawater.
While
in the case on surface enrichment in Li composition may be a result of silicate weathering
(if common in that area).
If
a water sample (TDS>1g/L) keeps its characteristics same to that of fresh
water (TDS<1g/L) it means the difference in TDS may be due to dissolving of
rock and soil for a longer duration.
Conclusions:
·
The increase of 𝛿7Li in groundwater and
surface water results from fractionation in clay sediments.
·
Li and its isotopes are useful in tracing of groundwater but
its fractionation may limit its use.
·
Li and O isotopes can be used in differentiating G.W salinity
sources.
·
Li/Cl & Li/Br can be used in suggesting marine salinity
source whether brine or seawater.


Comments
Post a Comment
Please provide your feedback.