Aquifer restoration Part-I
Introduction:
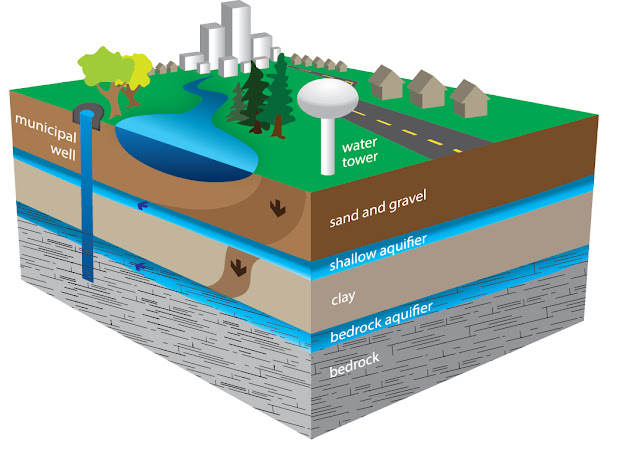
Pollution of water is a common topic and quite frequent word heard, but groundwater pollution is a little different word. Not the majority of people even know about there is such a word called "groundwater pollution".
So as the name suggests, it is pollution of the groundwater. The reason behind it is the mixing of chemicals in the groundwater due to the infiltration of the chemicals along with water supplied to fields for an increase in production. Although the majority of the chemical infiltrated doesn't meet ther groundwater. A part of the infiltrated chemicals is adsorbed by the layers of soil, another might be volatilized, a portion may be degraded by microbes and a part might be taken up by plants. So we see that there are a lot of reductions in the infiltrated chemicals which contribute positively, naturally in the reduction of groundwater pollution. Even though there is a part that goes and reaches and meets the groundwater.
Partitioning
Process
In this process, chemicals are partitioned by the soil itself into 2 parts
- The pollutant is adsorbed to the soil particle
- It can potentially be dissolved into pore water.
Adsorption
of the contaminant into the particulate form immobilizes contaminants and makes
them biologically unavailable in most cases.
Dissolved
(ionized) contaminants are mobilized with the soil pore water.
The
mobility of an organic chemical in soils is related to the octanol-water
partitioning coefficient, Kow.
Several mathematical formulations were given regarding this dissolved chemicals concept but Langmuir, Freundlich and linear isotherms are most widely accepted.
Several mathematical formulations were given regarding this dissolved chemicals concept but Langmuir, Freundlich and linear isotherms are most widely accepted.
The
Langmuir adsorption model is expressed as:
r =(Q0bCd)/(1+bCd)
r-adsorbed
concentration of the contaminant(μg/g)
The Cd-dissolved concentration of the contaminant in water or pore
water of soil(μg/L)
Q0-adsorption maximum at fixed temp.(μg/g)
b- constant related to the energy of net enthalpy of adsorption(L/μg)
The general form of the Freundlich isotherm is
r=K Cd (1/n)
where K and n are constant
If bCd in Langmuir isotherm is <1 or the exponent in the
Freundlich equation is close to 1 the isotherm becomes linear. The isotherm is
defined as
r = π Cd
π = b Q0
The total concentration of the
pollutant in the soil is then the sum of dissolved and particulate
concentration:
CT = θCd+rρbd
Where CT is the total concentration of the pollutant in
the soil(μg/L)
Retardation
In the sub-surface zone, chemicals move much slowly as compared in the surface zone. To make the calculations easier for the sub-surface zone, Retardation factor is introduced(R).Retardation factor is a term derived from the portioning coefficient an is the ratio of total chemicals to the dissolved chemicals:
R=Total Chemicals/Dissolved
Chemicals= (θCd+rρbd)/( θCd)
The chemicals move only in
dissolved phase and not in the adsorbed phase, hence the ratio of the velocity of
water to the velocity of solute can also be determined by R:
Vsolute = Vwater/R


Comments
Post a Comment
Please provide your feedback.